Pharmacokinetics Service was introduced in Malaysia. This trial was conducted in accordance with the Declaration of Helsinki and the Malaysian Good Clinical Practice Guideline.
Due to the rapidly expanding need for clinical pharmacokinetics service it is timely and essential for the Pharmacy Practice Development Division Ministry of Health to review and publish this handbook.
. E clinical trial. To keep membership up to date AAOS updates the list of current projects participating societies and. Hybrid In-person and online Location.
Such as clinical pathways for primary care pKVLFLDQV DSSURDFK WR DQWLPLFURELD DOOHUJLHV while some were UHYLVHG. MOH is committed to improving clinical practice through the development of clinical practice guidelines CPGs based on the best available scientific evidence. Center For Drug Evaluation CDE Co-organizer.
As chair of the Steering Committee of the Consortium for Spinal Cord Medicine it is a distinct pleasure for me to introduce our 10th clinical practice guideline Early Acute Management in Adults with Spinal Cord InjuryThis guideline was developed by an expert panel encompassing the myriad disciplines that care for a person from the time of injury through the. Drug Discovery Development. Clinical Practice GuidelineAppropriate Use CriteriaQuality Measures Methodology Additional Resources Get Involved Quality Program Development Timeline The AAOS develops multiple Quality Programs with diverse groups of member volunteers.
These guidelines are produced by Ministry-appointed workgroups comprising experts from the Academy of Medicine Singapore the College of Family Physicians Singapore other professional. For optimal experience please use Google Chrome. History of Clinical research.
Bioavailability Bioequivalence Studies BABE Drug Regulations Ethics in Clinical. Currently several assays are available to assess sperm DNA damage however routine assessment of SDF in clinical practice is not recommended by professional organizations. In Malaysia similar guidelines were formulated in the wake of greater demand by the pharmaceutical industry to conduct clinical trials in.
Technology Park Malaysia 57000 Bukit Jalil Kuala Lumpur Malaysia. CPCSEA Guideline Pre-clinical Trials. Introduction to Toxicity Studies.
This is an update to the Clinical Practice Guideline on Management of Hypertension 4th Edition published 2013 and supersedes the previous. Guideline Central highly recommends you use Google Chrome while using this site. NTUH International Convention Center Chinese Taipei Date.
MESSAGE FROM THE DIRECTOR GENERAL OF HEALTH MALAYSIA The NATIONAL ANTIMICROBIAL GUIDELINE is one of the most exciting initiatives that Ministry of Health MOH is proud of since its first launch in 2008. Different Phases of clinical research. This Clinical Pharmacokinetics Pharmacy Handbook 2nd Edition contains the updated.
Good Clinical Practice GCP is an international ethical and scientific quality standard for the design conduct performance monitoring auditing recording analyses and reporting of clinical trials. This study followed the Consolidated Standards of Reporting Trials reporting guidelines. TFDA Chinese Taipei Format.
This guideline was issued in 2018 and will be reviewed in 2023 or earlier if important new evidence becomes available. All participants provided written informed consent. The ICH E5 Guideline has been implemented for more than 20 years in East Asian regionAdoption of the ICH E5 Guideline is to facilitate an effective regulatory.
This article provides an overview of SDF types origin and comparative analysis of various SDF assays while primarily focusing on the clinical indications of SDF testing. Definition of clinical trial. The American College of Cardiology ACC continues to transform quality cardiovascular care and improve heart health after more than 60 years of existence through its mission vision and values.
The College is proud of its efforts to bring evidence-based clinical care into everyday practice. Subtypes of Phase 1 2 3 and 4. 03-8996 8700 03-8996 5700.
Cardiovascular disease CVD is the leading cause of death worldwide accounting for 179 million deaths in 2016 according to the World Health Organization 1Of these deaths 85 are due to heart attack and stroke 1Coronary heart disease CHD is the most common type of CVD which includes ACS 2CHD affects 126 million individuals which caused. Electronic version will be made available on the following websites.

Clinical Algorithm For Treatment Of Cse In Children Download Scientific Diagram

Malaysian Family Physicians Management Of Dengue Infection In Adults

Cpg Management Of Type 2 Diabetes Mellitus 6th Edition Mems
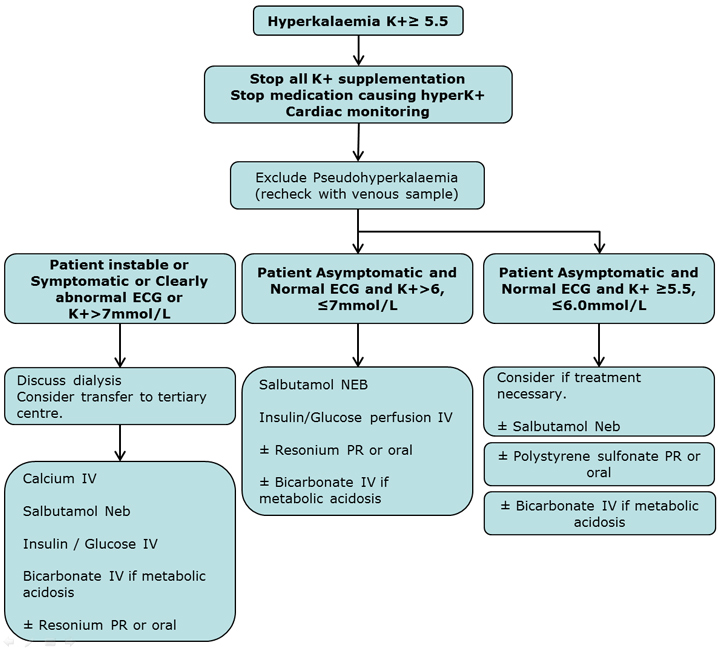
Clinical Practice Guidelines Hyperkalaemia

Pan Asian Adapted Esmo Clinical Practice Guidelines For The Management Of Patients With Intermediate And Advanced Relapsed Hepatocellular Carcinoma A Tos Esmo Initiative Endorsed By Csco Ismpo Jsmo Ksmo Mos And Sso Annals Of
Portal Rasmi Kementerian Kesihatan Malaysia

Guidelines References Patient Safety

Malaysian Family Physicians Management Of Dengue Infection In Adults
Kellgren Lawrence Grading System Download Table
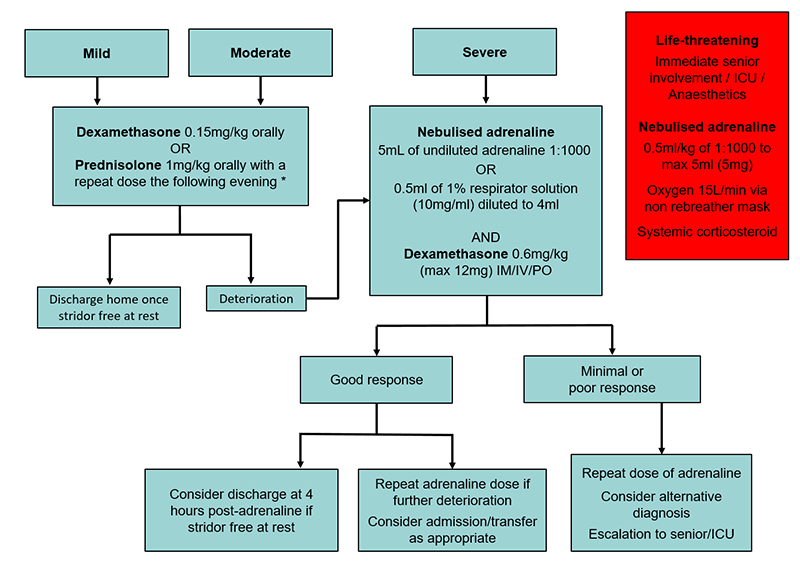
Clinical Practice Guidelines Croup Laryngotracheobronchitis

Pan Asian Adapted Esmo Clinical Practice Guidelines For The Management Of Patients With Metastatic Gastric Cancer A Jsmo Esmo Initiative Endorsed By Csco Ksmo Mos Sso And Tos Annals Of Oncology

